2025 |
||
| 3. |  | T. A. Hoang, H. D. Vu, D. Ngo, N. L. Mai, C. Doan, D. K. Tran, Y. Xiao, B. Rehm, Y. K. Yong, V. T. Dau Free-templated Microfabrication of Hydrogel Microneedles Using Electrohydrodynamic Technique for Transdermal Drug Delivery Conference The 29th International Conference on Miniaturized Systems for Chemistry and Life Sciences - Micro-Total Analysis Systems (uTas), Adelaide, 2 - 6 November, 2025, 2025. Abstract | BibTeX | Tags: Electrohydrodynamics, Microneedles, Pulsed voltage @conference{Hoang2025,This study presents a template-free electrostreching microfabrication method for hydrogel microneedles, targeting applications in wound healing and drug delivery. Precursor solutions of gelatin methacryloyl (GelMa) and hyaluronic acid methacryloyl (HAMA) were subjected to a high-voltage electric field, forming a hydrogel microneedle structure by undergoing UV-crosslinking and solvent evaporation. The fabricated microneedles demonstrated optimal geometries, i.e., height-to-base ratios and the ability to be grown in parallel. In vitro cytotoxicity test confirmed the biocompatibility of the microneedles, as cells were able to adhere and proliferate on their surfaces. Traditional transdermal drug delivery approaches, including hypodermic needles, topical creams, and transdermal patches, have significant drawbacks such as slow drug absorption and the painful and discomfort injection process [1], [2], [3]. Microneedles, which are arrays of micron-scale needles arranged in a small patch, offer an effective alternative solution by combining a non-invasive method and fast onset action to enhance patient compliance and potential for self-administration. Although there are various types of microneedles, most are fabricated using template-based methods such as moulding, lithography, or top-down micromachining, which require complicated processes in cleanroom facilities and limit design flexibility. To address these limitations, we report a template-free fabrication approach using electrohydrodynamic principles to produce microneedles directly on multiple substrate materials. Building upon prior work introduced in Small Methods [4], which focused on a proof-of-concept demonstration, this study extends the application to biocompatible hydrogels and evaluates the system’s scalability and safety for transdermal drug delivery. The method enables parallel microneedle fabrication and supports cell viability, highlighting its potential for practical therapeutic applications. An overview of microneedle fabrication is illustrated in Figure 1. A hydrogel solution containing GelMa, HAMA, and the photo-initiator LAP (Lithium phenyl-2,4,6-trimethylbenzoylphosphinate) was prepared and dispensed as a droplet onto a substrate positioned between the two high-voltage electrodes. Under the applied pulsating electric field , the droplet was elongated and stretched into a conical shape (Taylor-cone shape). The droplet was then exposed to UV light to initiate hydrogel photo-polymerisation, which solidified the droplet’s outer layer. By the subsequent air-dry process, the solvent completely evaporates, resulting in a solid microneedle. This method enabled parallel microneedle formation, as shown in Figure 2, where five microneedles were simultaneously grown with consistent form and geometry. Figure 3 demonstrates the in-situ adaptability of the method, showcasing its ability to grow microneedles directly on various commercially available adhesive patches. GHMa-1, -2, and -3 samples exhibited desirable geometries, with height-to-base ratios from 1.90 to 3.23 (Table 1). Biocompatibility of the microneedle was validated by culturing cells (RAW 264.7) in direct contact with the microneedle, where cell adhesion and proliferation were observed (Figure 4). These findings underscore the potential of this technique for scalable and flexible fabrication of biocompatible hydrogel transdermal drug delivery platforms. |
| 2. |  | N. L. Mai, Y. K. Yong, T. A. Hoang, T. H. Vu, H Vu, V. C. Doan, D. Cai, T. X. Dinh, D. V. Dao, V. T. Dau Fabrication of Microneedles by Pulsating In Situ Dried Electrostretching for Transdermal Drug Delivery Journal Article In: Small Methods, vol. 9, pp. 2500183, 2025, (The paper's cover art has been selected as the frontispiece of the journal, Oct 2025). Abstract | Links | BibTeX | Tags: Electrohydrodynamics, Microneedles, Pulsed voltage @article{Mai2025,This paper introduces a novel pulsating in situ dried electrostretching (PIDES) technique for the fabrication of microneedles (MNs) for transdermal drug delivery. This method utilizes pulsed voltage to induce electrohydrodynamic forces that stretch and freeze a polymer droplet into a conical shape with a micrometer-scale tip. With the effects of solvent evaporation, the polymeric droplet is in situ stretched into a conical shape and solidified, transforming into a sharp MN, suitable for transdermal drug administration. Penetration and mechanical tests confirm that the MNs possess sufficient sharpness and strength for effective skin penetration applications. Additionally, curcumin loading and in vitro release tests with different concentrations demonstrate the MNs' ability to carry drugs and exhibit effective controlled release profiles. These findings highlight PIDES as a promising, low-cost, and simple approach for the development of painless and efficient transdermal drug delivery systems. |
| 1. | 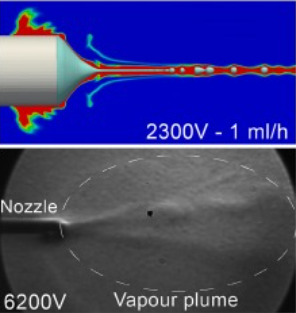 | N. L. Mai, T. H. Vu, H. Vu, C. Doan, Y. K. Yong, T. X. Dinh, D. V. Dao, V. T. Dau Evaporation in electrohydrodynamic atomisation: A numerical and experimental investigation Journal Article In: International Communications in Heat and Mass Transfer, vol. 165, Part B, pp. 109079, 2025. Abstract | Links | BibTeX | Tags: Electrohydrodynamics, Evaporation @article{Mai2025b,In this paper, the influence of evaporation in electrohydrodynamic atomization (electrospray) is numerically and experimentally investigated. The simulation was performed utilizing the Taylor-Melcher's leaky-dielectric model and the d2 Law to simulate thermophysical processes in electrospray. Experiments were conducted to validate the numerical approach and to investigate the influence of evaporation on particle morphology. Results by simulations are consistent with experiments, showing agreement in both Taylor-cone captured by high-speed camera and vapour field visualized by Schlieren imaging. Experiments on different solvents suggest major impacts of interelectrode distance on the residual solvent on the collected particles. However, these impacts were less significant on particle size and morphology particularly when using solvents with medium to low volatility. Particle size is found to increase with temperature and solvent volatility, confirming the correlation reported in literature. Moreover, evaporation was determined to have limited effect on the overall shape of the Taylor-cone and spray jet, due to inherently high vapour concentration around the nozzle vicinity. These contributions will improve the understanding of evaporation process in electrospray and offer useful guidelines for optimizing the technique, particularly in situations where evaporation is a key factor controlling particle size and minimizing harmful residue. Keywords: Numerical methods; Evaporation; Electrospray; Electrohydrodynamic atomization; Experiments; Particle morphology |
2025 |
||
| 3. |  | Free-templated Microfabrication of Hydrogel Microneedles Using Electrohydrodynamic Technique for Transdermal Drug Delivery Conference The 29th International Conference on Miniaturized Systems for Chemistry and Life Sciences - Micro-Total Analysis Systems (uTas), Adelaide, 2 - 6 November, 2025, 2025. |
| 2. |  | Fabrication of Microneedles by Pulsating In Situ Dried Electrostretching for Transdermal Drug Delivery Journal Article In: Small Methods, vol. 9, pp. 2500183, 2025, (The paper's cover art has been selected as the frontispiece of the journal, Oct 2025). |
| 1. |  | Evaporation in electrohydrodynamic atomisation: A numerical and experimental investigation Journal Article In: International Communications in Heat and Mass Transfer, vol. 165, Part B, pp. 109079, 2025. |
